1. Introduction
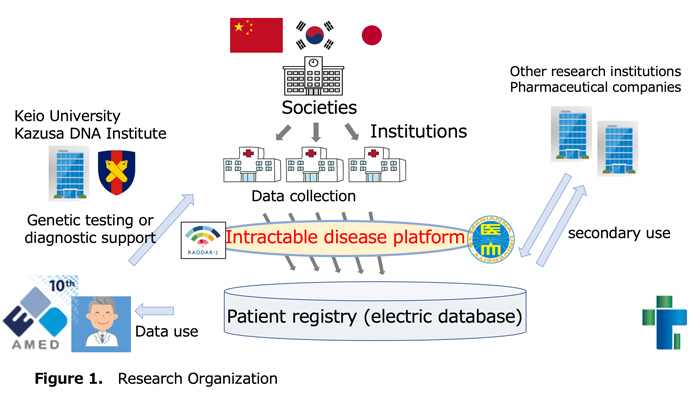
This is clinical research targeting patients with congenital lipoid adrenal hyperplasia conducted primarily by the AMED Practical Research Project LCAH Research Team (Figure 1).
This research has been reviewed by the Ethics Committee of Tokyo Metropolitan Children's Medical Center for its validity from ethical and scientific perspectives, and is being conducted with the approval of the Ethics Committee and permission from each institution.
This research is registered in the clinical trial registration system operated by the University Hospital Medical Information Network (UMIN) Center (UMIN Clinical Trial ID: UMIN000050445).
2. Purpose
The main purpose of this research is to collect data on the clinical course and medical care of as many patients as possible with congenital lipoid adrenal hyperplasia continuously and long-term, to predict and avoid complications specific to this condition, and to optimize treatment.
3. Significance
Advancing this research may realize the following in the future:
1) Advancement and acceleration of research
By collecting clinical information from more patients and sharing it among healthcare professionals and researchers, understanding of diseases that were previously unclear will advance, potentially leading to prediction of future symptoms, avoidance of complications, optimization of current treatments, and development of new treatments and drugs.
2) Increased opportunities to participate in clinical trials
Research on medical policies tailored to individual constitutions is progressing worldwide. By registering in the patient registry (patient database) for congenital lipoid adrenal hyperplasia, opportunities to be involved in the development of testing and treatment methods suited to individuals may increase.
3) Access to latest disease information
LCAH registry research is conducted by a group of specialists including pediatric endocrinologists, adult endocrinologists, urologists, obstetricians and gynecologists, and epidemiologists. Because the latest information about this condition is gathered from Japan and abroad, participating in this research may make it easier to receive the latest information about this condition from medical institutions.
4. Methods
(1) Implementation method (Figure 2)
In this research, attending physicians will regularly provide "clinical information" such as patient symptoms and test results identified through routine medical care through a system on the Nanbyo Platform* every six months.
* The Nanbyo Platform is one of the public databases, a secure information integration infrastructure that consolidates clinical information and information from biological samples collected by research groups for designated intractable diseases of the Japan Agency for Medical Research and Development (AMED) and the Ministry of Health, Labour and Welfare.
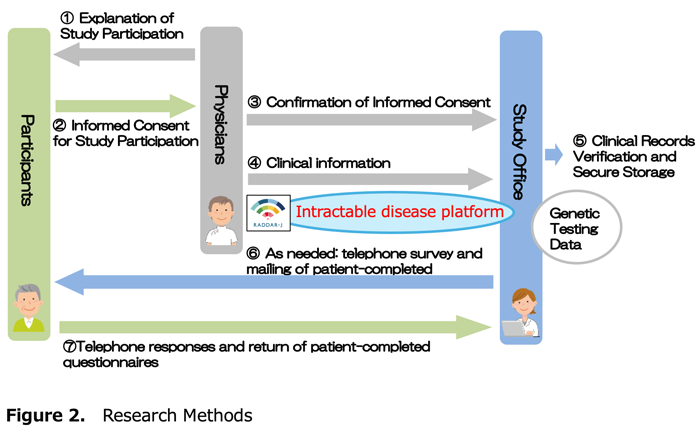
(2) Cooperation items
Collecting clinical information regularly and completely leads to high-quality research. Not only attending physicians, but also research office staff may directly contact patients by telephone or questionnaire (patient-completed) for follow-up.
The following clinical information will be provided:
Standard items: name, contact information, date of birth, assigned sex, race, birth information, diagnosis, presence of designated intractable disease patient certification, date at onset, date at diagnosis, medical institution and department, quality of life survey (EQ-5D-5L), outcome
Semi-standard items: educational background, medical history, living situation, preferences, genetic testing, family history, pregnancy/delivery information, complications, parental information, birth information
Additional items: physical findings (height, weight, abdominal circumference, blood pressure, etc.), clinical tests (blood chemistry tests, endocrinological tests), spot urine tests, semen analysis, gonadal ultrasound, bone mineral density, treatment status (medications, dosages), SF-36 quality of life survey*
*SF-36 quality of life survey:
Targets patients aged 18 and over. A QR code will be provided to each patient, and they will input information at home every six months.
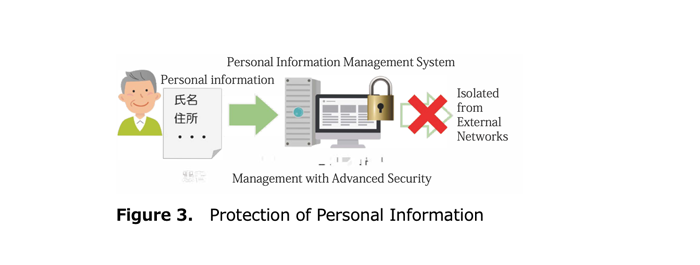
(3) Handling of personal information (Figure 3)
This research collects information that can identify individuals, such as names and telephone numbers. This information is encrypted and stored distributed across multiple more secure servers (personal information management system) separate from servers where other clinical information is registered. Therefore, even if equipment is taken out or there is external intrusion into servers, it is extremely difficult to extract information that can identify patients.
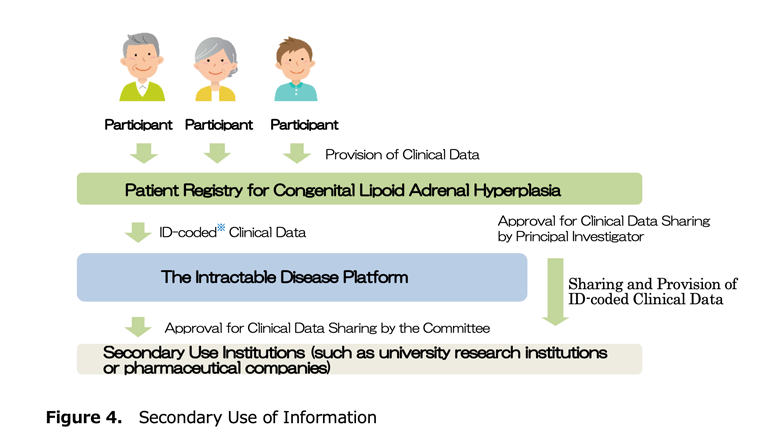
(4) Secondary use of information (Figure 4)
By consenting to participate in this research, you also consent to sharing and providing clinical information to the Intractable Disease Platform. This research is also linked with the Intractable Disease Platform, and clinical information of patients managed by ID is ultimately shared with the Intractable Disease Platform along with data from patients in registry research for other diseases.
Provided clinical information may be shared with other research institutions in Japan and abroad (called secondary use institutions). When providing clinical information to secondary use institutions, after obtaining approval from the principal investigator of this research regarding whether the research plan of the destination is scientifically and ethically appropriate, whether there is no disadvantage to patients, and whether the content complies with ethical guidelines for medical research,it is reviewed by the Intractable Disease Platform operating committee, and only secondary use institutions approved there can use the clinical information. Secondary use institutions are prohibited from using clinical information beyond the approved scope. Also, information that can identify individuals is not provided to secondary use institutions.
5. Benefits and disadvantages for participants
(1) Benefits of participating in this research
It may help predict prognosis, avoid complications, and optimize treatment for yourself and other patients.
It may become easier to receive the latest information on newly discovered pathophysiology and treatments related to this condition. Opportunities to participate in clinical trials may increase.
A QUO card worth 2,000 yen is provided to research participants as a token of appreciation for their cooperation.
(2) Disadvantages of participating in this research
There is no new physical or mental burden arising from participating in this research.
Because clinical information is provided within the scope of routine medical care, there is no new cost burden beyond the insurance medical burden (regular outpatient visit fee).
6. Research structure
Research is being conducted at the following institutions:
Tokyo Metropolitan Children's Medical Center, Keio University, Kyoto University, Oita University, Fukushima Medical University, Tokushima University, Hamamatsu University School of Medicine, Osaka Women's and Children's Hospital, St. Marianna University School of Medicine Yokohama City Seibu Hospital, Niigata University Medical and Dental Hospital, Aichi Children's Health and Medical Center, Ota Memorial Hospital, Iseikai International General Hospital, Nagoya City University Hospital, National Center for Child Health and Development, Saitama City Hospital, Kyushu University Hospital, Shizuoka City Shimizu Hospital, Kanazawa University, Tokyo Dental College Ichikawa General Hospital, Shinshu University, Jichi Medical University Children's Medical Center Tochigi, Tokyo Women's Medical University, Hyogo Prefectural Children's Hospital, Hokkaido University, Shizuoka Red Cross Hospital, Ichinomiya Municipal Hospital, Toyama University, Tokyo Metropolitan Tama Hokuba Medical Center, National Hospital Organization Kanazawa Medical Center, Toyohashi Municipal Hospital, Yamagata University, Kitasato University, University of Occupational and Environmental Health, Japan, The University of Tokyo, and others.
7. Contact information
If you have any concerns about the content of this research, please feel free to contact us. Please use the "Contact Link" on the top page.
8. Request for cooperation in clinical research
We request your cooperation in clinical research.